Identify the Products of the Following Acid-base Reaction:
B CH3COOH NO2 NH02 CH3COO. None of these 117.

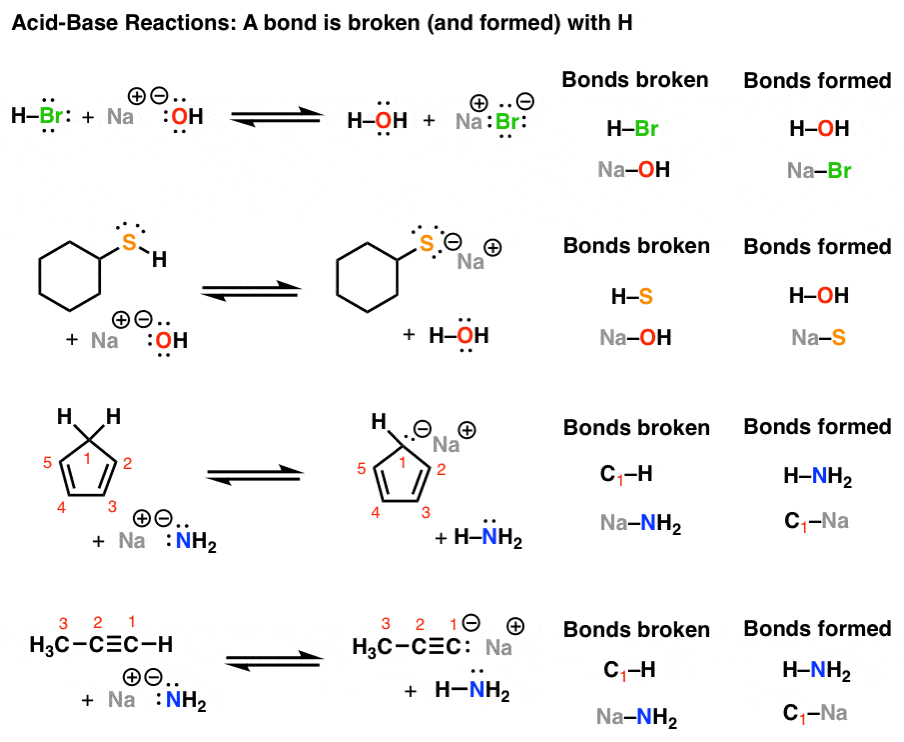
Acid Base Reactions In Organic Chemistry Master Organic Chemistry
A HClO4aq H2Ol H3Oaq ClO4aq b.
. Textbook solution for ORGANIC CHEM-SMSTDGDELL-WACCESS 8th Edition Bruice Chapter 154 Problem 7P. Consider the following reactions of acetic acid CH 3 COOH the organic acid that gives vinegar its characteristic taste. O Check whether the reactants are an acid and a base.
Write the products of the following Grignard reactions. HCO3- is the conjugate base. Draw electron-dot structures for the reactants and products and use the curved arrow notation Section 1516 to represent the donation of a lone pair of electrons from the Lewis base to the Lewis acid.
Acid-base precipitation or redox Only one reaction type applies to each reaction and all three should be used. The reaction has been given as. For the following reaction identify the Lewis acid.
Identify the acid base conjugate acid and conjugate base for the following reactions. None of these 118. The Brønsted-Lowry acid-base theory has several advantages over the Arrhenius theory.
The products of a neutralization reaction are usually. A NH3 HI NH4 I. Phphenyl a benzene ring attached to a carbon chain HNO3 H2O F H3O H3C CH3 O OH PhCOO HCl H2CO3 OH H2O OH H NH3 HCC 4.
For the following reaction identify the Lewis base. A 2 HClO4aq CaOH2aq 2H2Ol CaClO42aq B Fes 2AgNO3aq 2 Ags FeNO32aq C Cs O2g CO2g D MgSO4aq BaNO32aq MgNO32aq BaSO4s E None of. Fill in the reactants or products for the following acid-base reactions.
Express your answer as part of a chemical equation. 1Mg 1 Br MgBr. Label each species as an acid or a base.
In the following acid-base reaction how would you identify the acid base and their conjugate acids and bases. Carbon dioxide and oxygen. For the following reaction identify the Lewis base.
X AY AX Y. Identify all of the phases in your answer. An acid and a base.
Thus option C is correct. NH4aq CN- -- NH3aq HCNaq NH4 and NH3 is a conjugate acid base pair. For the following reaction identify the acid and base reactants and the conjugate acid and conjugate base products.
A mathrm HNO_ 2mathrm H_ 2 mathrm O rightleftharpoons mathrm H_ 3 mathrm O mathrm NO_ 2 - b mathrm HPO_ 4 2. The Grignard reagent is a good source of alkyl group which acts as a nucleophile and attacks on the. RESOURCES Practice Problem 0935a Identify the products of the following acid-base reaction.
A blood ph 74 b A 1 M solution of hydorchloric acid. Chemistry questions and answers. For the acid-base reaction label the acid the base the conjugate acid and the conjugate base.
Identify the acid on the left side of the following equation and identify its conjugate base on the right side. When in doubt write complete Lewis structures. Redox reactions were briefly introduced in gr10.
NH4 HCO3- -- NH3 H2CO3. Recall that the Arrhenius theory defines an acid as any species that increases the concentration of H H 3 O in solution. In this chapter learners will explore acid-base reactions and redox reactions.
112 Identify the conjugate acid-base pairs in the following reaction. CN- and HCN is a conjugate acid base pair. Identify each reactant and product in the following chemcial reactions as a acid a base or neither.
For the following reactions a write the balanced reaction equations and b IDENTIFY the type of reaction as ONE of the following. 2 Classify the following samples from most acidic to most basic. Arrange the species in each reaction as conjugate acid-base pairs.
Which of the following is an acid base reaction. The X has been highly reactive than Y replaces Y in the compound and forms the compound AX. H2CO3 aq H2O l ------- H3O aq HCO3aq Group of answer choices H2O is the acid.
Check whether the products are an acid and a base. H3O is the conjugate base H2CO3 is the acid. The concepts of acids bases reduction oxidation and oxidation numbers are all introduced here.
In the acid-base reaction H3O OH- 2H2O the H3O ion transfers an to the OH- ion. Check if the ions of two compounds exchange places. HNO3aq NH3aq NO3aq NH4aq.
An acid-base reaction can therefore be described as an transfer process. NH3aq H2Ol NH4aq OH-aq. 2 HH a b 3 NHCI H0.
Keep in mind that in line-angle formulas hydrogens are not shown. 0403 LC Which of the following steps can be used to identify an acid-base reaction Check if one element replaces another element in a compound. The single replacement reaction can be identified when there has been the replacement of one element with another element in the compound.
Chemistry Acids and Bases Conjugate Acids and Conjugate Bases. Predict the products of the following acid-base reactions and predict whether the equilibrium lies to the left or to the right of the equation. NH4 is the conjugate acid and NH3 is the conjugate base.
We have step-by-step solutions for your textbooks written by Bartleby experts. 2 HH a b 3 NHCI H0. The following list provides a summary of the topics covered in this chapter.
For the following reaction identify the Lewis acid. 1539 The reaction of PCl 4 with Cl is a Lewis acidbase reaction. HCO3 is the conjugate base H2O is the acid.
SkW0902 SkW0902 Skill 1903 Checkpoint Н Check Checkpoint Checkpoint H- L and Checkpoint H Li and Checko Skil 0922 Checkpoint _L and Í - L and I Skill 0929 Skill 0930 Glem 0922d em 0932 blem 0934 blem OT 350 slem. None of these 119. Identify the conjugate acid-base pairs in each of Conjugate Acid-Base Pairs.
SOLVEDIdentify each reactant and product in the following chemical reactions as a Bronsted-Lowry acid a BronstedLowry base or neither. Arrange the species in each reaction as conjugate acid-base pairs.

Introduction To Acid Base Reactions Master Organic Chemistry

Predicting Products Of Acid Base Reactions Youtube

How To Determine The Position Of Equilibrium For An Acid Base Reaction Chemistry Steps